
Somatic Cancer
Liquid Biopsy
SHOP PRODUCTSAccuracy at extreme levels of sensitivity is crucial to the clinical utility of highly multiplexed NGS-based liquid biopsy assays. These tests demand purpose-built, patient-like reference materials to verify assay sensitivity and performance before delivering critical patient results.
SeraCare’s patent-pending circulating tumor DNA (ctDNA) technology ensures the most patient-like ctDNA sizing, clinically-relevant variant coverage, and improved library complexity to accelerate liquid biopsy NGS assay development, validation, and routine clinical implementation.
Comprehensive solutions across all phases of the NGS workflow
ctDNA v2 & ctDNA CompleteTM
- Comprehensive ctDNA reference materials containing 40 clinically relevant pan-cancer variants (ctDNA v2) or 25 solid tumor variants including three CNVs (ctDNA Complete).
- Patient-like sized blend of contrived variant constructs and background wild-type DNA.
- Available in a range of allelic frequencies (Afs) from 0.1% to 5% as purified DNA Mix or encapsulated DNA in synthetic plasma
Seraseq® ctDNA MRD Panel
- An ultra-low allelic frequency ctDNA reference material for development and limit of detection (LoD) validation of NGS-based MRD assays
- Derived from a diseased cell line harboring a high number of somatic variants, its SNP-matched normal cell line, and additional therapeutically relevant biosynthetic DNA variants
- Tumor and normal DNA is blended to four tumor fractions (0.5%, 0.05%, 0.005% and 0%), fragmented and sized to mimic patient ctDNA; all blends are characterized by NGS
Better assays and more thorough validations
- Confidently evaluate your assay's concordance with tissue-based tests using allele frequencies up to 5%
- ctDNA-like size distribution and efficient library incorporation enhance NGS workflow performance and data quality
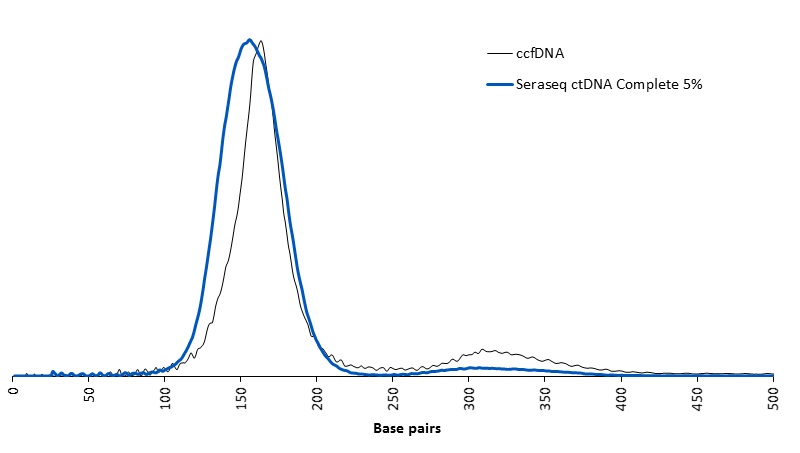 |
Representative DNA fragment size distribution for the Seraseq ctDNA Complete™ Mutation Mix AF5% versus natural circulating cell-free DNA (ccfDNA)
|
Ensure assay sensitivity and accuracy
- Determine your assay’s LOD with orthogonally validated allele frequencies down to 0.1%
- Confidently establish your assay’s ability to detect SNVs, INDELs, CNVs, and SVs with reference materials featuring the broadest coverage of all variant types
- Expert-designed content features up to 40 clinically relevant variants associated with diagnosis, disease progression and resistance monitoring, and therapeutic selection
Gene List
| AKT1 ■ ● |
BRCA1 ● |
ERBB2 ■ ● |
GNA11 ■ |
KIT ■ ● |
NCOA4-RET ■ ● |
PTEN ■ |
| ALK ● |
BRCA2 ● |
ERBB2 CNV ● |
GNAQ ■ |
KRAS ■ ● |
NPM1 ■ |
RET ■ |
| APC ■ |
CD74-R0S1 ● |
FGFR3 ■ |
GNAS ■ |
MET CNV ● |
NRAS ■ ● |
SMAD4 ■ |
| ATM ■ |
CTNNB1 ■ |
FLT3 ■ |
IDH1 ■ |
MPL ■ |
PDGFRA ■ |
TP53 ■ |
| BRAF ■ ● |
EGFR ■ ● |
FOXL2 ■ |
JAK2 ■ |
MYC CNV ● |
PIK3CA ■ ● |
TPR-ALK ■ ● |
ctDNA v2 ■ ctDNA complete ●

