SARS-CoV-2 Molecular Solutions
FULL VIRAL GENOME COVERAGE FOR ASSAY VERIFICATION AND ONGOING PERFORMANCE MONITORING
In support of industry efforts to develop SARS-CoV-2 testing formats compatible with alternative sample types, we now offer AccuPlexTM SARS-CoV-2 reference material in a synthetic oral fluid matrix in addition to our standard matrix compatible with nasopharyngeal swab sourced samples. All of our Quality Solutions are designed to challenge the entire molecular test procedure ensuring clinical laboratories can have confidence in their SARS-CoV-2 assay results.
AccuPlex™ SARS-CoV-2 Verification Panel is optimized for assay verification at installation by documenting test performance along the assay’s range enabling laboratories to establish lower limits of detection, perform assay comparisons, and evaluate staff proficiency. The product contains positive materials including the full SARS-CoV-2 viral genome, and negative materials targeting the human RNase P gene.
|
Product Description |
Material Number |
Region |
Pack Size |
Concentration |
|
|
|
|
Full Genome |
Positive 1 |
1 x 3 mL |
100,000 copies/mL |
Not for In Vitro Diagnostic Use. Research Use Only.
AccuPlex™ SARS-CoV-2 Reference Material is designed to measure day-to-day performance of the assay, providing both a positive and a negative reference solution. The product is available in two versions: the Reference Material Kit covers the CDC and WHO consensus sequences; the Molecular Controls Kit is expanded to include the full SARS-CoV-2 viral genome (Table 1). All products also include negative reference materials targeting the human RNase P gene.
|
Product Description |
Material Numbers |
Region |
Pack Size |
Concentration |
|
AccuPlexTM SARS-CoV-2 |
ORF1a, RdRp, E, N |
Positive 5 x 1.5 mL Negative 5 x 1.5 mL |
5,000 copies/mL 5,000 copies/mL (RNase P gene) |
|
|
AccuPlexTM SARS-CoV-2 |
Full Genome |
*Not for In Vitro Diagnostic Use. Research Use Only.
**For In Vitro Diagnostic Use. CE-IVD marked.
Click here for bulk quantities or custom requests

Click here to learn more about SARS-CoV-2 Serology Solutions.
SARS-CoV-2 Quality Solutions:
- Non-infectious and replication deficient, enables safe handling in contrast to viral samples
- Fully-extractable with a real viral protein coat; superior to “naked” transcribed RNA
- Compatible with assays targeting CDC and WHO consensus sequences (Table 1)
- Includes negative reference material for targeting sequences for the human RNAse P gene
- 2 year stability at 2 - 8°C
- Customizable to sequences of interest to meet unique assay design requirements
Table 1: Regions included in AccuPlex Coronavirus (SARS-CoV-2) Positive Reference Material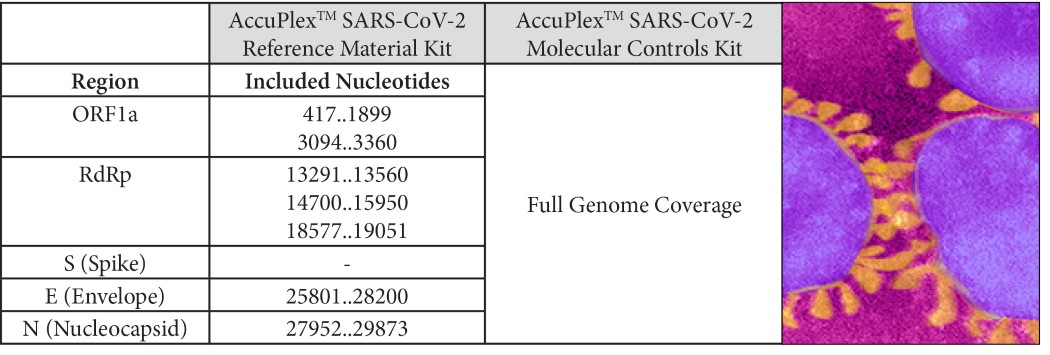 Numbers refer to sequence of GenBank NC_045512.2
Numbers refer to sequence of GenBank NC_045512.2
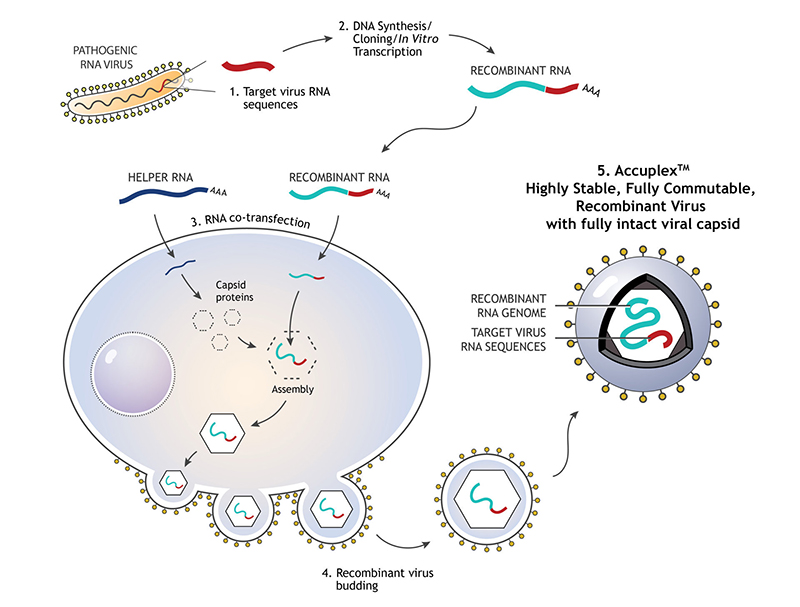
LGC SeraCare’s AccuPlex recombinant material serves as a true full process molecular control for your diagnostic assays. Compatible with multiplexed RT-PCR and NGS-based assays, AccuPlex custom recombinant materials are constructed with a replication-deficient mammalian virus, producing a safe, non-infectious material (Figure 1 ). With a protein coat and lipid bilayer, these mammalian virus-based reference materials resemble the complexity of virus targets found in true patient samples.
FIGURE 1: 1) RNA sequence from the pathogenic virus of interest is chosen. 2) DNA synthesis and cloning occur to produce the recombinant RNA. 3) Recombinant RNA and helper RNA are co-transfected into the mammalian cells, allowing the encapsulation of recombined RNA. 4) Exocytosis of the mature enveloped non-infectious and replication deficient RNA virus containing the assay target RNA sequence of interest.

LGC, Biosearch Technologies Probes and Primers for SARS-CoV-2
Commercially available probe and primer kits released for distribution by CDC testing results. Additional probes and primers following other protocols published by WHO are also available.
|
Product Description |
Catalog Number |
Size |
|
2019-nCoV CDC-qualified Probe and Primer Kits for SARS-CoV-2 |
1000 rxns |
|
|
US CDC 2019-nCoV ValuPanel Reagents* |
10,000-100,000 rxns |
|
|
Charité 2019-nCoV BBQ-650 ValuPanel Reagents* |
1,000-100,000 rxns |
|
|
Charité 2019-nCoV BHQ ValuPanel Reagents* |
1,000-100,000 rxns |
Bead-based nucleic acid purification chemistry that enables automated high-throughput extraction and purification of high quality RNA.
|
Product Description |
Catalog Number |
Size |
|
sbeadex viral RNA purification kit* |
960 5000 10000 |
|
|
sbeadex viral RNA purification kit, no proteinase K* |
960 5000 10000 |
LGC, Biosearch Technologies RT-PCR Reagents for SARS-CoV-2
High-performing, lyo-compatible master mix for sensitive pathogen detection and an optimized reverse transcriptase system for the production of full-length cDNA.
|
Product Description |
Catalog Number |
Size |
|
RapiDxFire 1-step RT-qPCR System* |
0.125 mL 1.25 mL 1 mL 10mL |