SeraCare has partnered with industry experts to design, develop, and commercialize a portfolio of immuno-oncology reference materials to support the harmonization and validation of NGS assays measuring tumor mutational burden (TMB) and microsatellite instability (MSI). Seraseq TMB and MSI reference materials help labs identify patients most likely to respond to immunotherapy treatment with greater confidence.
Features and benefits
- Industry’s first tissue TMB (tTMB) and blood TMB (bTMB) reference materials to support different modes of TMB analysis.
- Wide range of TMB scores to challenge assay performance across different tumor types and clinical cut-offs.
- TMB reference materials are extensively characterized using orthogonal analysis by whole exome sequencing (WES) and NGS targeted panels.
- Tumor-only and tumor-normal matched MSI reference materials support NGS-based MSI analysis as well as gold-standard qPCR-based methods.
Tumor Mutational Burden (TMB) Solutions
Tissue TMB
Seraseq TMB reference materials were developed from matched tumor-normal human cell lines in both purified gDNA and FFPE formats. These cell lines are derived from diseased patients and their SNP-matched peripheral blood lymphoblastoid normal cell lines.
Tissue TMB reference materials are characterized by WES and a TMB analysis pipeline (tumor-normal mode) to determine their empirical TMB scores. Complementary analysis by targeted NGS assays was performed by participating members of the SeraCare TMB Working Group.
Validate and harmonize TMB measurements in different sample types:
- Support TMB analysis and scoring harmonization between gold-standard WES and targeted NGS panels
- Available as 100% purified tumor / normal set DNA or 30% tumor FFPE format.
- TMB scores 7, 9 13, 20 and 26
The Seraseq gDNA TMB Reference Panel Mix, in a purified gDNA format, spans a wide range of WES-validated TMB scores between 5 and 50, requiring only one matched-normal sample for all scores, all in one kit. It can be used to guide TMB score assessment using targeted panels, especially around values that may be clinical decision points for different tumor types.
Download the Product Sheet to learn more, and Data Sheet for the details of the WES workflow and complementary analyses by targeted NGS assays.
Blood TMB
Seraseq blood TMB (bTMB) reference materials are developed based on the primary tumor-normal matched cell lines, and have been evaluated by industry-leading targeted NGS assays. To account for the typical relative abundance of tumor cells in blood, blood TMB reference materials are blended at different tumor fractions for analysis by ctDNA NGS panels using a tumor-normal workflow.
Accelerate the development and validation of ctDNA-based TMB assays
- TMB scores of 7, 13, 20 and 26, as determined using the TruSight Oncology 500 (TSO500) ctDNA panel.
- Derived from human diseased and matched-normal cell lines
- Genomic DNA blended at three tumor fractions – 0.5%, 2% and 0% (WT), fragmented and sized to mimic patient ctDNA
- Available as purified ctDNA ready for NGS library preparation
View the Datasheet for more details about the blood TMB assessments.
MicroSatellite Instability (MSI) Products
We have developed tumor-normal matched MSI reference materials (available as purified DNA at AF 5% or AF 20%) to support development, verification of assay LoD and implementation of molecular and genomic assays interrogating tumor MSI status at specific mono- and dinucleotide repeats. Refer to the following table for examples of 5 clinically validated biomarker loci.
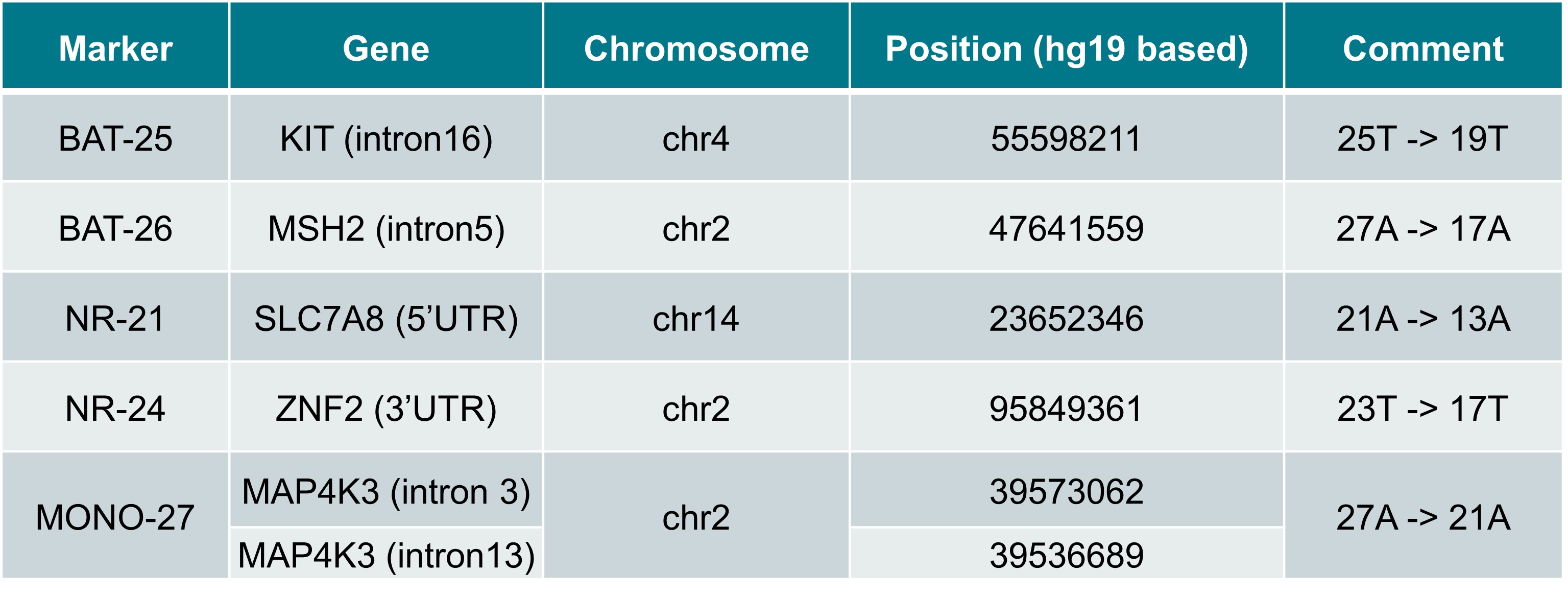
Note: There is ambiguity in the literature on the MONO-27 locus so two constructs are included in the product to ensure compatibility
The tumor only MSI-High products in purified DNA and FFPE formats enable validation of MSI-High calls using NGS assays including MSI scoring.
The table below highlights the TSO500 assay analysis of MSI status of the Seraseq MSI-High products (gDNA and FFPE).
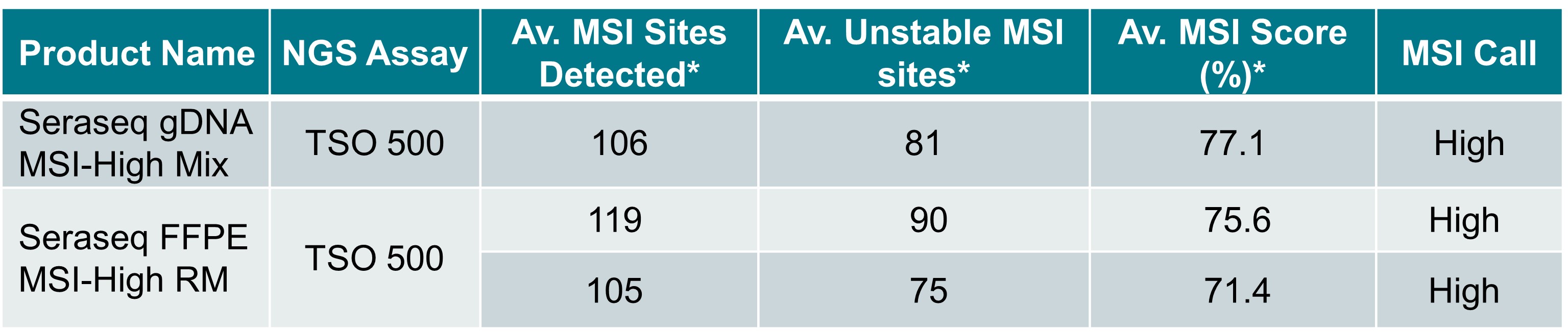
*MSI measurements are from replicate runs using the TSO 500 assay. MSI score is the ratio of unstable MSI sites to the total number of sites detected (expressed as a percentage). The value must be >20% for an MSI-High result.
Download the Data Sheet to view how Seraseq gDNA MSI reference standards have been analyzed using qPCR/CE, digital PCR and targeted NGS assays .
References
1. Diana M. Merino, Laura M. Yee, Lisa M. McShane, Mathew G. Butler, Vincent Funari, Mathew D. Hellmann, Ruchi Chaudhary, Shu-Jen Chen, Wandjuh Chen, Jeffrey M. Conroy, David Fabrizio, Laura E. MacConaill, Aparna Pallavajjala, Arnaud Papin, Mark Sausen, Victor J. Weigman, Mingchao Xie, Ahmet Zehir, Chen Zhao, and P. Mickey Williams, TMB Standardization by Alignment to Reference Standards: Phase 2 of the Friends of Cancer Research TMB Harmonization Project, Poster #268, 2019 ASCO Meeting, Chicago, https://meetinglibrary.asco.org/record/172797/abstract
2. Butler, M.G., et. al., Characterization of Tumor-Normal Cell Line Pairs for TMB Standardization, Poster ST084, 2019 AMP Conference, Baltimore, MD.
3. Butler, M.G., et. al., Development of Reference Materials for Blood Tumor Mutational Burden Measurement, Abstract #1982, 2020 AACR Annual Meeting, Virtual II, June 2020.
4. Diana Merino Vega, Alignment of TMB measured on clinical samples: Phase 2B of the Friends of Cancer Research TMB Harmonization Project, 2020 AACR Virtual I Meeting, https://www.focr.org/sites/default/files/AACR_2020_TMB.pdf
5. Eun-Ang Raiber-Moreau, et. al., Development and Validation of Blood Tumor Mutational Burden Reference Standards”, Poster #60, 2020 Society for Immunotherapy in Cancer (SITC), Dec 2020.

