Seraseq NGS Technology

Developing, optimizing, and monitoring next-generation sequencing (NGS) assays is a difficult task. Successful assays require accuracy throughout the entire process, from sample DNA purification and quantitation, to library construction and template preparation, through bioinformatics parameters and variant annotation. Reference materials play a key role as “gold standards” and allow assays to be robustly developed, thoroughly validated and ensure accuracy and standardization of results.
Flexible, highly-multiplexed NGS products
SeraCare’s Seraseq NGS technology offers scalability, flexibility, and commutability, which is responsive to the needs of in vitro diagnostics (IVD) manufacturers and clinical laboratories. By using highly characterized cell lines and engineered biosynthetic DNA targets (figure below), SeraCare can produce highly-multiplexed reference standards for the demands of a wide range of targeted sequencing panels, including RNA and DNA formats, that interrogate a breadth of genomic events.

Innovative and patient-like biosynthetic technology
Production of biosynthetic DNA with sequences that occur rarely in patient samples solves the problem of sourcing these rare materials in limited supply. In addition, the use of biosynthetic DNA and engineered cell lines creates the ability to adjust individual allele frequency, gene amplifications, gene fusions, or fetal fraction percentages to a given commonly accepted limit of detection, or the ability to customize a set of variant allele frequencies or fetal fraction percentages to user requirements.

Manufacturing methodology
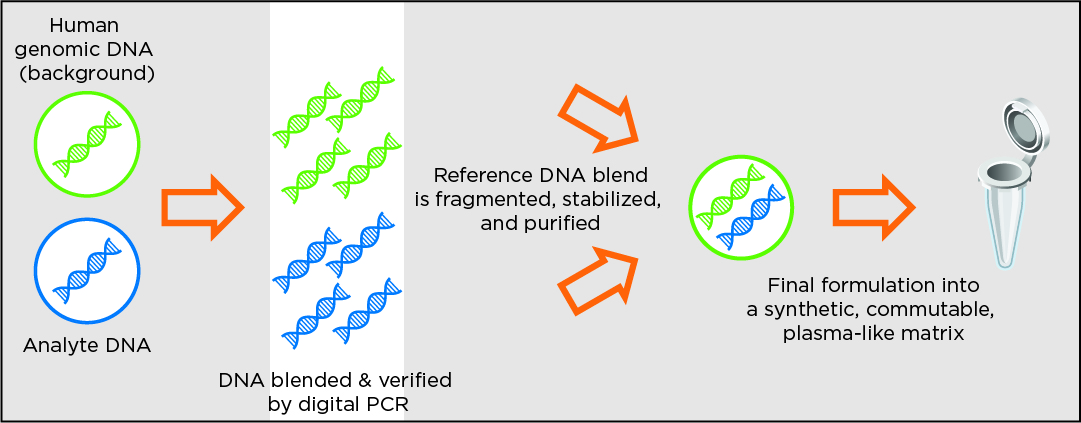
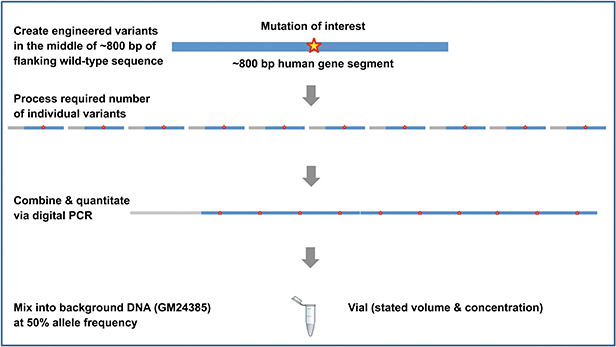
The Seraseq family of somatic cancer and inherited disease products are manufactured using a patented and innovative methodology developed by SeraCare (figure below). Target variants of interest are synthetically manufactured, further processed as necessary, and blended with a single well-characterized background material (GM24385) at the required allele frequency. Digital PCR assays are used as the gold standard methodology to precisely modulate and control the allele frequency. The products are then configured as necessary to the final format.
Design method for products in the gDNA format
Full-process reference materials provide maximum usability
The use of stable circulating cell-free DNA in synthetic plasma-like material and formalin-fixed, paraffin-embedded (FFPE) cell lines enables Seraseq products to serve as full-process reference materials, monitoring the entire workflow. These reference standards can be handled in an identical fashion as a patient sample in procedures including:
-
Nucleic acid extraction
-
Quantitation
-
NGS library construction
-
Template preparation
-
Sequencing
-
Data analysis
Design method for products in synthetic plasma reference material format
The below methodology applies to the cfDNA-focused full-process reference material products such as circulating tumor DNA (ctDNA) and reproductive health products for trisomies and euploid.