AccuPlex Recombinant Virus Materials

The Safest, True Full-Process Control
The key to successful development, validation, and implementation of molecular diagnostic assays is the availability of abundant, non-infectious, and consistently-manufactured reference materials.
AccuPlex provides the power of flexibility to create custom solutions to your most challenging reference material needs.
Compatible with multiplexed RT-PCR and NGS-based assays, AccuPlex custom recombinant virus materials are constructed with a replication-defective mammalian virus, producing a safe, non-infectious material (Figure 1). With a protein coat and lipid bilayer, these mammalian virus-based reference materials resemble the complexity of virus targets found in clinical samples.
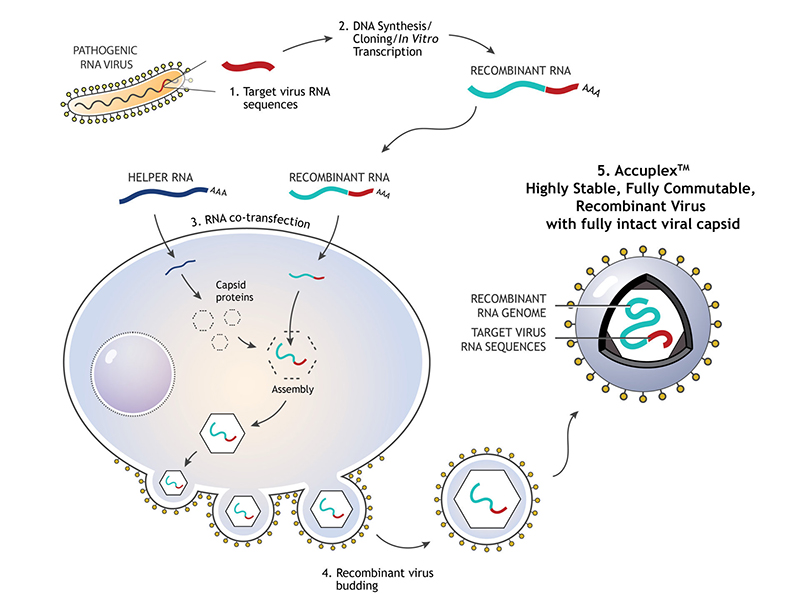
Figure 1:
- RNA sequence from the pathogenic virus of interest is chosen
- DNA synthesis and cloning is performed to produce the recombinant RNA
- Recombinant RNA and helper RNA are co-transfected into the mammalian cells, allowing the encapsulation of recombined RNA
- Exocytosis of the mature enveloped RNA virus with the RNA sequence of the virus of interest occurs
AccuPlex Solves Development Challenges

- Non-infectious and replication-defective for safe and effective handling of positive material
- Fully-extractable with a real viral protein coat to serve as a full-process control
- Accepts multiple sequences from same or different RNA viruses, customizable to your sequences of interest
- Digital PCR (ddPCR) QC step allows for a wide range of titer levels, offers flexible concentrations
- Compatible with multiple sample matrices including DBS, buffer, serum, and semen
- Stability studies confirm product is stable at 4°C, room temperature, and elevated temperatures
Patient-Like Material Evaluates Entire Workflow
Unlike other technologies that package the viral RNA into a bacteriophage, the AccuPlex recombinant virus releases the viral genome at a similar rate as that of wild-type mammalian pathogenic virus during the nucleic acid sample preparation step. AccuPlex recombinant virus material mimics a real clinical sample across the entire workflow, serving as a full-process control.
- Replication deficient with a flexible sequence design, as opposed to cultured virus options
- True full-process control with a complex viral structure, unlike MS-2 bacteriophage
Your Partner for Custom Recombinant Controls
If you’re challenged with finding safe, non-infectious controls for your emerging viral disease assay, partner with SeraCare for custom AccuPlex recombinant virus material, whether DNA or RNA-based. Utilizing your target sequence of interest and product specifications, we will develop a custom solution to accelerate your development and implementation timelines.


