PGT-A Reference Materials

Preimplantation genetic testing (PGT) or preimplantation genetic screening (PGS) both refer to the same process of testing embryos prior to their transfer into a patient during an in vitro fertilization (IVF) cycle. The aim of the method is to screen embryos for genetic anomalies and improve the odds of a successful pregnancy. There are three types of PGT testing that can be performed either individually or in combination:
- PGT-A - testing for chromosomal aneuploidies
- PGT-SR - testing for chromosomal structural rearrangements
- PGT-M - testing for monogenic disorders
Click here to download the Seraseq® PGT-A Reference Materials product sheet detailing assay validation and daily-run QC material for pre-implantation genetic testing (PGT-A).
Assay Development
Are you working on a new PGT-A assay? You will need to:
- Test and optimize the protocol
- Test and determine sensitivity and specificity
- Test LOD, range of fetal fractions
- Monitor manufacturing and lot quality
Our materials are proven to expedite assay development, optimization and manufacturing.
Validation and Verification
Are you licensing a 3rd party test or expanding to new locations? Let us help you:
- Meet regulatory guidelines
- Develop a consistent benchmark
- Ensure the test performance is as expected
- Validate new reagent lots or protocol changes
We help labs quickly and effectively validate, verify and train your staff.
Routine Run QC
Do you want to save time, money and increase confidence in your results? We offer reference materials that:
- Proactively monitor assay and instrument performance
- Expedite troubleshooting
- Ensure accuracy of daily results
- Monitor the full process by using patient-like materials
Labs running our samples routinely are able to rapidly detect and respond to sample failures and ensure accuracy.
Material obtained for PGT is small and very precious. It has to deliver accurate results to guide the decisions on embryo transfer. Most common way to obtain testing material is an embryo biopsy, done most commonly at the blastocyst stage (day 5). An alternative method relies on a non-invasive PGT by sampling embryo culture media (niPGT-A). Due to a small amount of genetic material obtained in the process, the first step of PGT is whole genome amplification (WGA).
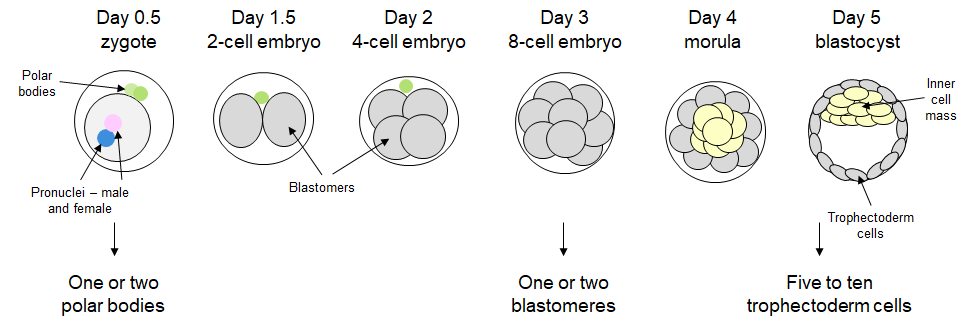
Figure 1. Early embryonic development with three most stages used for embryo biopsies.



