AccuPlex™ Flu A/B and RSV Verification Panel
Details
Resources
To expedite your order, if you already have an e-commerce account, please proceed with placing your order via our website. If you do not currently have an eCommerce account, please email your purchase order to CustomerService@Seracare.com or contact us at 1.800.676.1881. If you need to set us up as a new vendor, please reference our W-9.
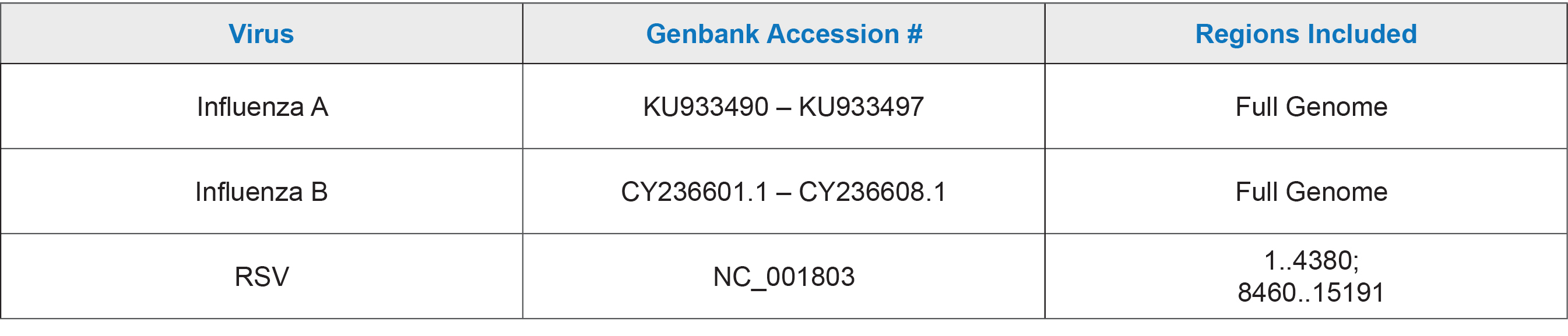
AccuPlex™ Flu A/B and RSV Verification Panel is formulated for use with multiplex test methods that can detect influenza A/B and respiratory syncytial virus. Included are three different concentrations of positive reference material as well as negative reference material directed against the human RNase P gene. The panel is optimized for assay verification at installation by documenting test performance along the assay’s range enabling laboratories to establish lower limits of detection, perform assay comparisons, and evaluate staff proficiency.

Click here for bulk quantities or custom requests
LGC SeraCare’s Flu A/B and RSV AccuPlex solution:
- Non-infectious and replication deficient, enables safe handling in contrast to viral samples
- Fully-extractable with a real viral protein coat; superior to “naked” transcribed RNA
- 2 year stability at 2 - 8°C